| Catalog Number: | CL-CDX-05 |
| CAS No.: | 144790-28-3 |
| Mol.F: | C16H17N3O5S |
| Mol.W: | 363.4 |
| Inv. Status: | Custom Synthesis |
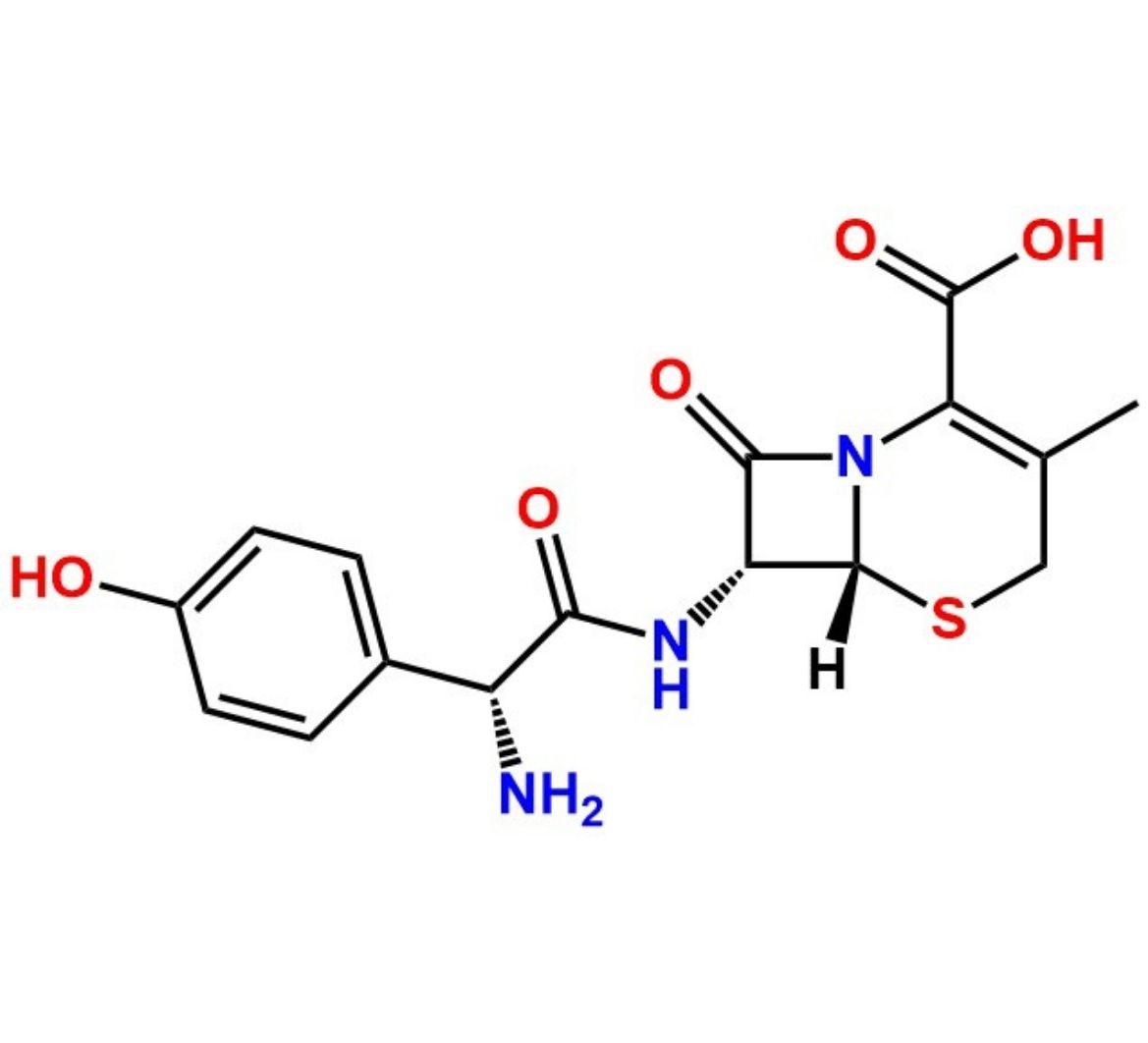
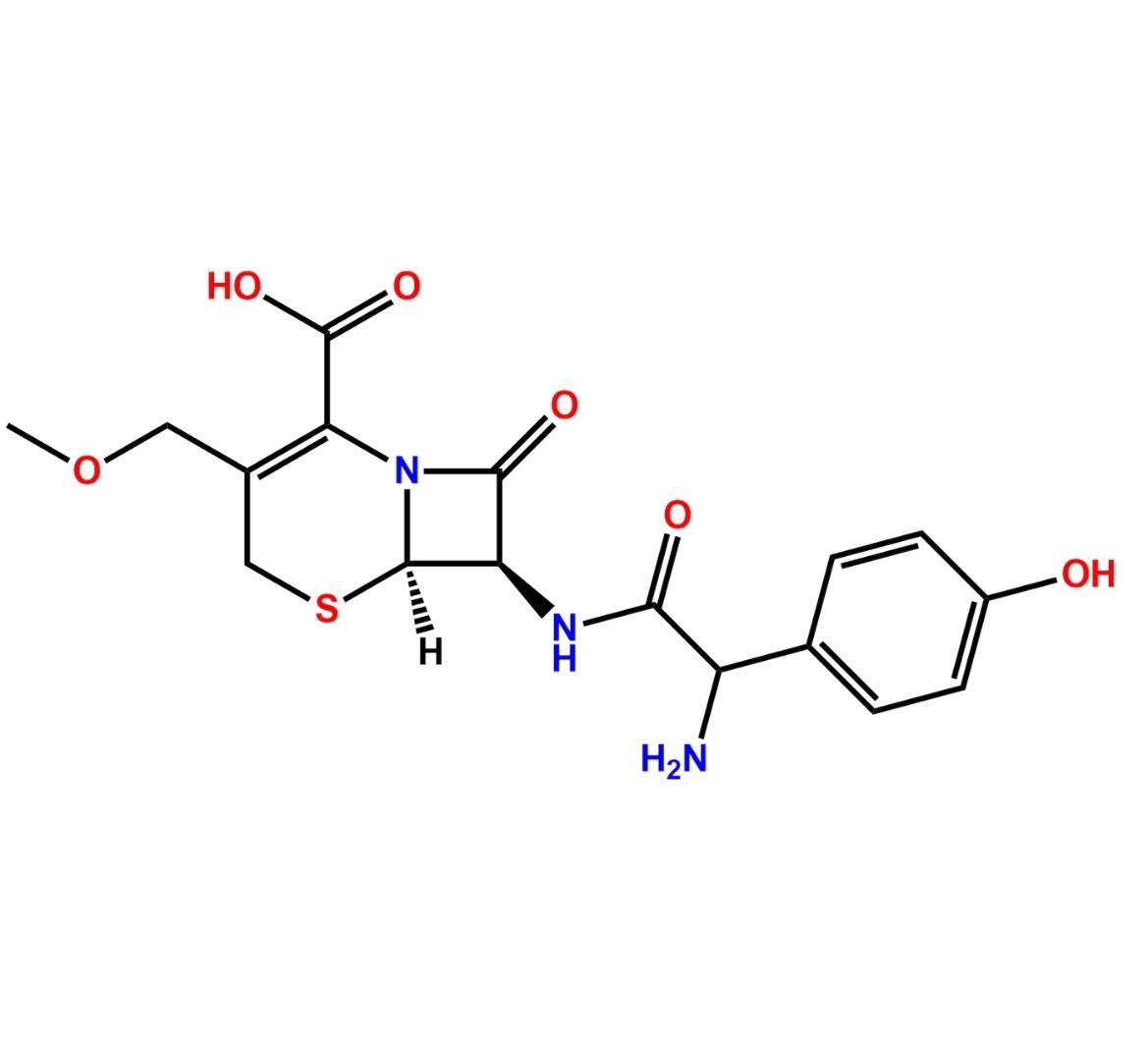
Chemical Name: (6R,7R)-7-[[(2S)-2-Amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid ; (6R,7R)-7-[(S)-2-Amino-2-(4-hydroxyphenyl)acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (as per USP).
Country of Origin: India
Product Category:Cefadroxil Impurities
Cefadroxil EP Impurity D is identified by CAS No. 144790-28-3 and is chemically known as (6R,7R)-7-[[(2S)-2-Amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid ; (6R,7R)-7-[(S)-2-Amino-2-(4-hydroxyphenyl)acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (as per USP).
Cefadroxil EP Impurity D is provided with comprehensive characterization data in accordance with regulatory guidelines. It is suitable for use in analytical method development, method validation (AMV), Quality Control (QC) applications for Abbreviated New Drug Applications (ANDA) and commercial production of Cefadroxil.
Page Keywords: Buy Cefadroxil EP Impurity D | Purchase Cefadroxil EP Impurity D | Cefadroxil EP Impurity D suppliers | Cefadroxil EP Impurity D manufacturers | Cefadroxil EP Impurity D price | Cefadroxil EP Impurity D Importer | Cefadroxil EP Impurity D Exporter

 English
English
 Arabic
Arabic
 Chinese (Simplified)
Chinese (Simplified)
 French
French
 Japanese
Japanese
 Portuguese
Portuguese
 Russian
Russian
 Spanish
Spanish


.jpg)





.jpg)

.jpg)
.jpg)
.jpg)
.jpg)
